
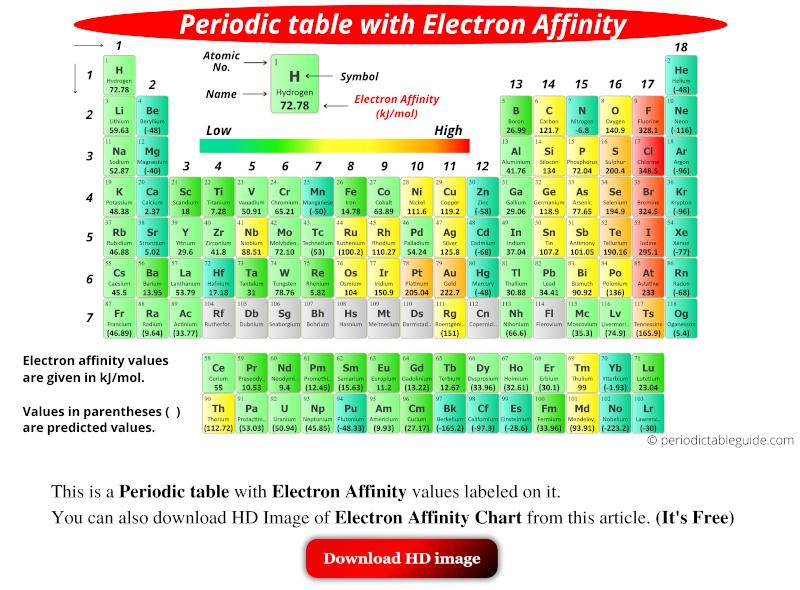

A trend of decreasing electron affinity down the groups in the periodic table would be expected since the additional electron is entering an orbital farther away from the nucleus. Chlorine has the highest electron affinity while mercury has the lowest.Įlectron affinity generally increases across a period (row) in the periodic table, due to the filling of the valence shell of the atom. The electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values.
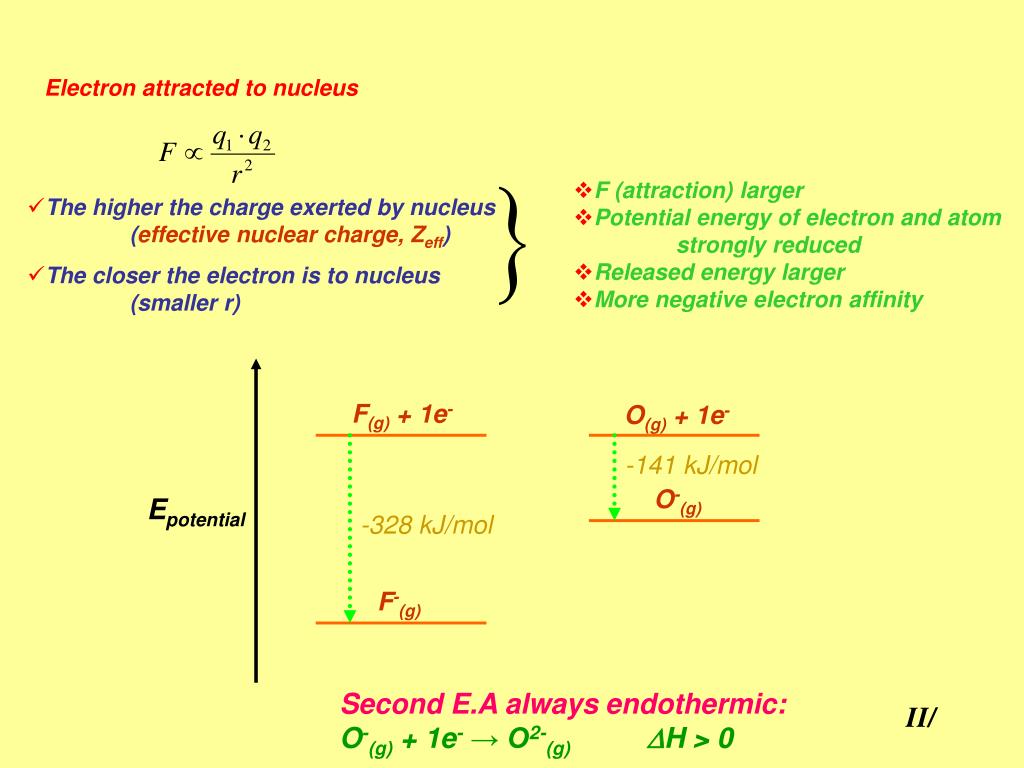
Atoms, such as Group 7 elements, whose anions are more stable than neutral atoms have a higher electron affinity. Generally, nonmetals have a more positive electron affinity than metals. This can be shown for the chloride ion formation below: The electron affinity (E ea) of a neutral atom or molecule is defined as the amount of energy released when an electron is added to it to form a negative ion. Electron affinity is the energy change that occurs when a neutral atom gains an electron.


 0 kommentar(er)
0 kommentar(er)
